HPV-Q Comprehensive Real-Time PCR Kit
Example report includes genotype mapping for 28 HPV types, internal control results, and amplification curves for result verification.


Comprehensive detection of high-risk and low/medium-risk HPV genotypes at the point of care, empowering community health programs with expanded cervical cancer screening capability.
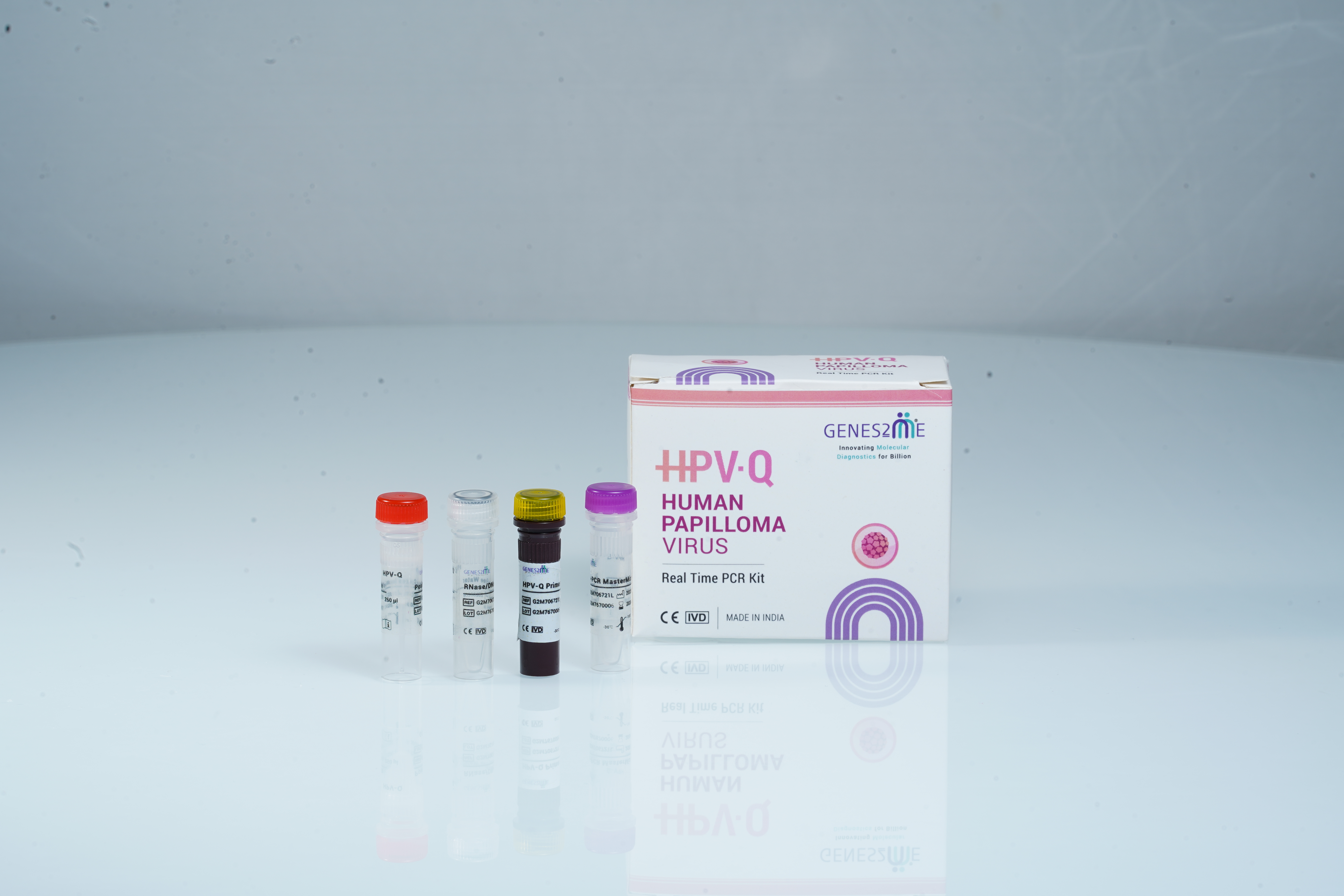
The HPV-Q Comprehensive Real-Time PCR Kit (POCT) is a complete in vitro diagnostic solution designed for point-of-care detection of 28 HPV genotypes, covering both high-risk and low/medium-risk types, associated with cervical and other anogenital infections. Using multiplex Real-Time PCR technology and lyophilized reagent formulation,this kit provides rapid, sensitive, and broad-spectrum HPV detection without the need for cold storage — ideal for field-based and large-scale screening initiatives.
| Targets Covered | |
|---|---|
| 28 HPV Genotypes: Inclusive of all high-risk, low-risk, and medium-risk types. | |
| HPV16 & HPV18: Differential detection for clinical risk stratification. | |
| Internal Control (RNaseP): Monitors sample adequacy and assay integrity. | |
Government & NGO Health Missions:- Large-scale HPV detection programs
Mobile Diagnostic Units:- On-site testing without cold chain dependency
Research & Epidemiology Centers:- Genotype distribution studies

Example report includes genotype mapping for 28 HPV types, internal control results, and amplification curves for result verification.


| Commercial Name | Cat No. | Pack Size | Compatible Instruments |
|---|---|---|---|
| HPV-Q Real-Time PCR Kit | G2M803921R | 24T | OnePCR POC device |
| HPV-Q Real-Time PCR Kit | G2M803921R | 48T | Rapi-Q POC Device |
Reliable, portable, and accurate HPV detection technology, All-in-one, field-deployable HPV genotyping solution.
Detects 28 genotypes across all risk categories — from high-risk oncogenic to low-risk benign types.
Ideal for large-scale cervical cancer elimination initiatives aligned with WHO goals.
No cold chain required; simple handling and rapid setup.
High sensitivity and specificity in diverse screening environments.
Download useful documents and technical information for the HPV-Q Comprehensive.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.