Drug-resistant tuberculosis (TB) remains a major challenge for global TB control. Resistance can arise from MDR/XDR strains or factors like delayed diagnosis and inadequate treatment. Timely detection is critical, yet traditional drug susceptibility testing (DST) using culture methods takes weeks due to the slow growth of Mycobacterium tuberculosis, increasing the risk of MDR and XDR-TB spread. While rapid molecular tests exist, their high-cost limits accessibility in resource poor regions.
Genes2Me’s NGS-based TB panel offers a breakthrough delivering fast, accurate detection for Mycobacterium Tuberculosis Complex and drug-resistant TB enabling early intervention, optimized therapy, and improved patient outcomes. Using high throughput targeted sequencing approach directly from clinical specimens and eliminating the need for culture, this assay is designed to map 100 kb region of the M. tuberculosis genome for 75 genes and associated mutation sites, as well as SNP loci. Get a complete drug-resistance profile for all major first and second line anti-TB drugs (including drug resistance, MDR, Pre-XDR, and XDR) with the Genes2Me TB NGS Assay. Powered by advanced hybridization capture-based target enrichment, this assay delivers high-accuracy detection of resistance mutations, enabling rapid, comprehensive insights for effective treatment planning.
Drug Resistance Profiling: With the G2M TB NGS Assay, unlock a complete drug-resistance profile for all major first and second line anti-TB drugs. This advanced panel also includes coverage for the latest WHO approved nitroimidazole antibiotics Delamanid and Pretomanid used in BPaL and BPaLM treatment regimens. The table lists major anti-TB drugs grouped by treatment lines and WHO recommended categories:
| First Line | Rifampicin | |
| Isoniazid | ||
| Ethambutol | ||
| Pyrazinamide | ||
| Second Line | Group A | Bedaquiline |
| Linezolid | ||
| Moxifloxacin | ||
| Levofloxacin | ||
| Group B | Clofazimine | |
| Cycloserine | ||
| Group C | Delamanid | |
| Ethionamide | ||
| Amikacin | ||
| Streptomycin | ||
| Para-aminosalicylic acid | ||
| Others | Pretomanid | |
| Capreomycin | ||
| Kanamycin |
*The panel’s performance is from the Illumina platform.
The G2M Tuberculosis Panel Reveals a Comprehensive Gene-Drug Resistance Network for Precision Therapy
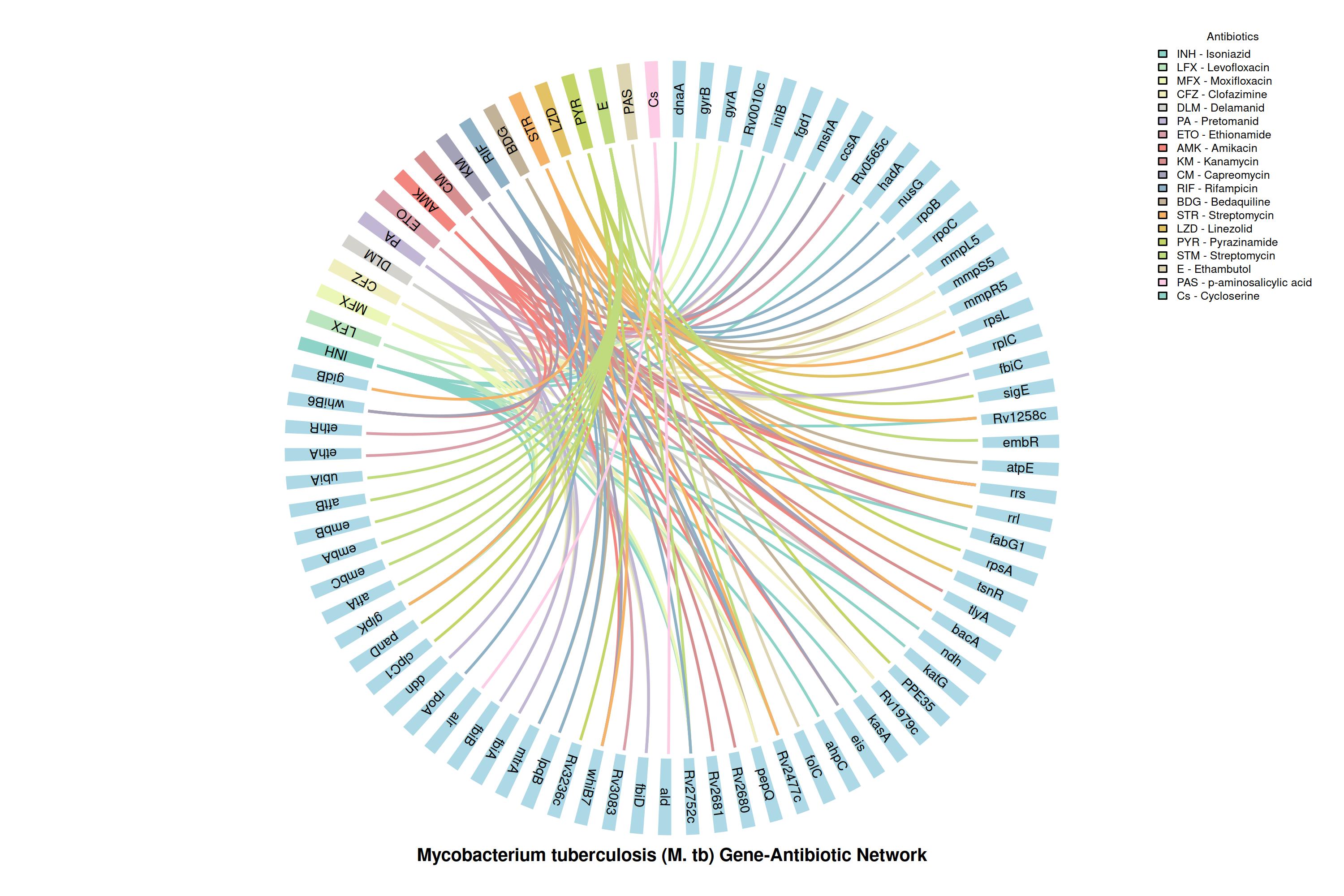
The chord diagram visualizes connections between Mycobacterium tuberculosis resistance genes and anti-TB drugs, including first line, second line, and WHO-approved regimens. This mapping highlights critical genetic determinants of MDR, Pre-XDR, and XDR-TB, enabling rapid detection and guiding precision treatment strategies by the G2M panel.
Consistent Gene Coverage and Depth Across TB Samples Confirms Assay Reliability
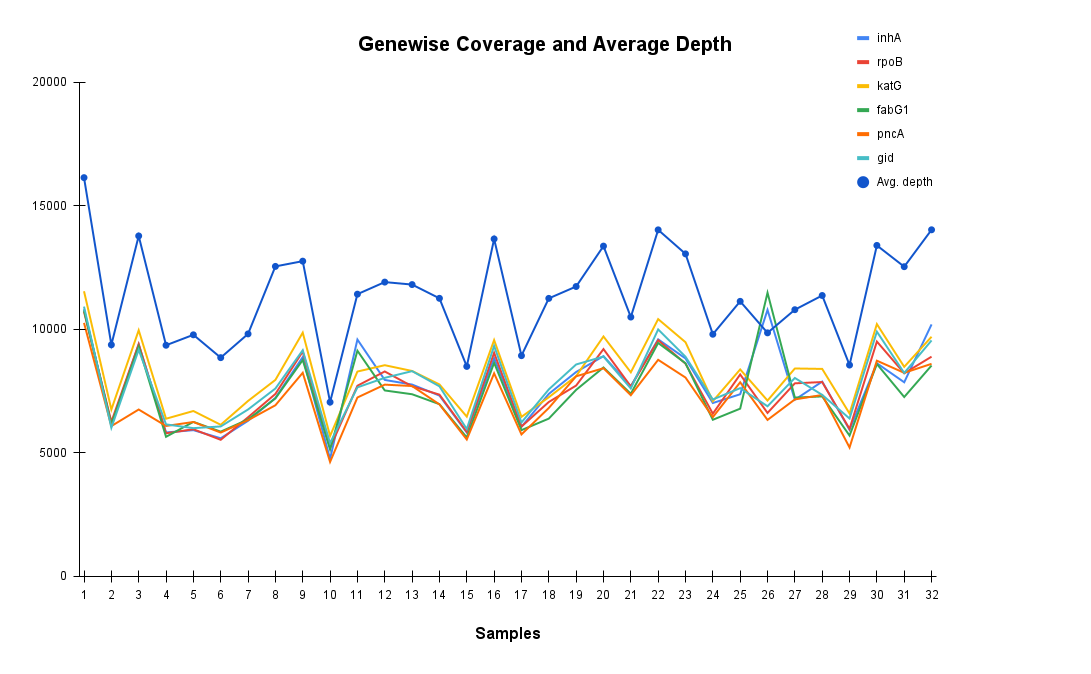
The plot illustrates gene wise coverage and average sequencing depth across 32 TB samples for critical resistance-associated genes (inhA, rpoB, katG, fabG1, pncA, gid). Coverage remains consistently high across all targets, while average depth (blue line) demonstrates robust sequencing performance. Accurate detection of resistance mutations ensures uniformity supporting reliable diagnosis and tailored therapeutic planning for MDR, Pre-XDR, and XDR-TB.
Widespread TB Drug Resistance Highlights Importance of Comprehensive Profiling
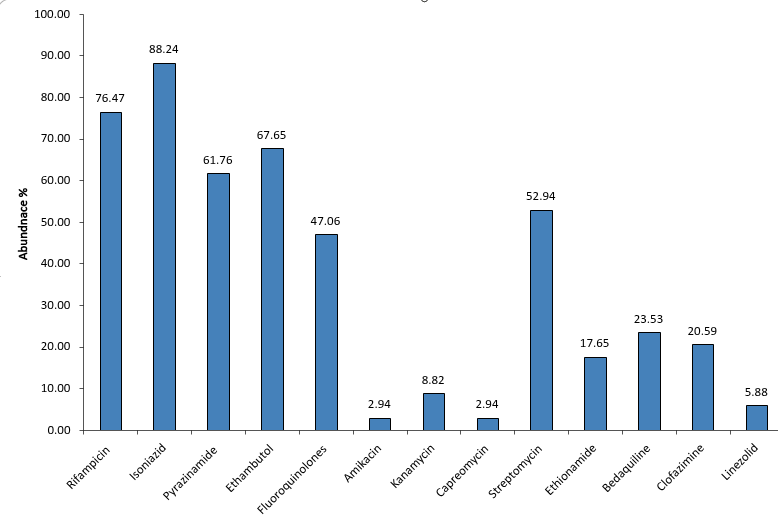
The graph illustrates the relative abundance of drug resistance across first-line and second-line anti-TB medications. Higher resistance is observed for primary drugs such as Isoniazid, Rifampicin, Pyrazinamide, and Ethambutol, followed by Fluoroquinolones. Second-line drugs including Amikacin, Kanamycin, Capreomycin, Streptomycin, Ethionamide, Bedaquiline, Clofazimine, and Linezolid show variable resistance patterns. This highlights the importance of comprehensive resistance profiling for effective TB treatment planning.
G2M TB Panel Provides Full Resistance Insight for Optimized Care
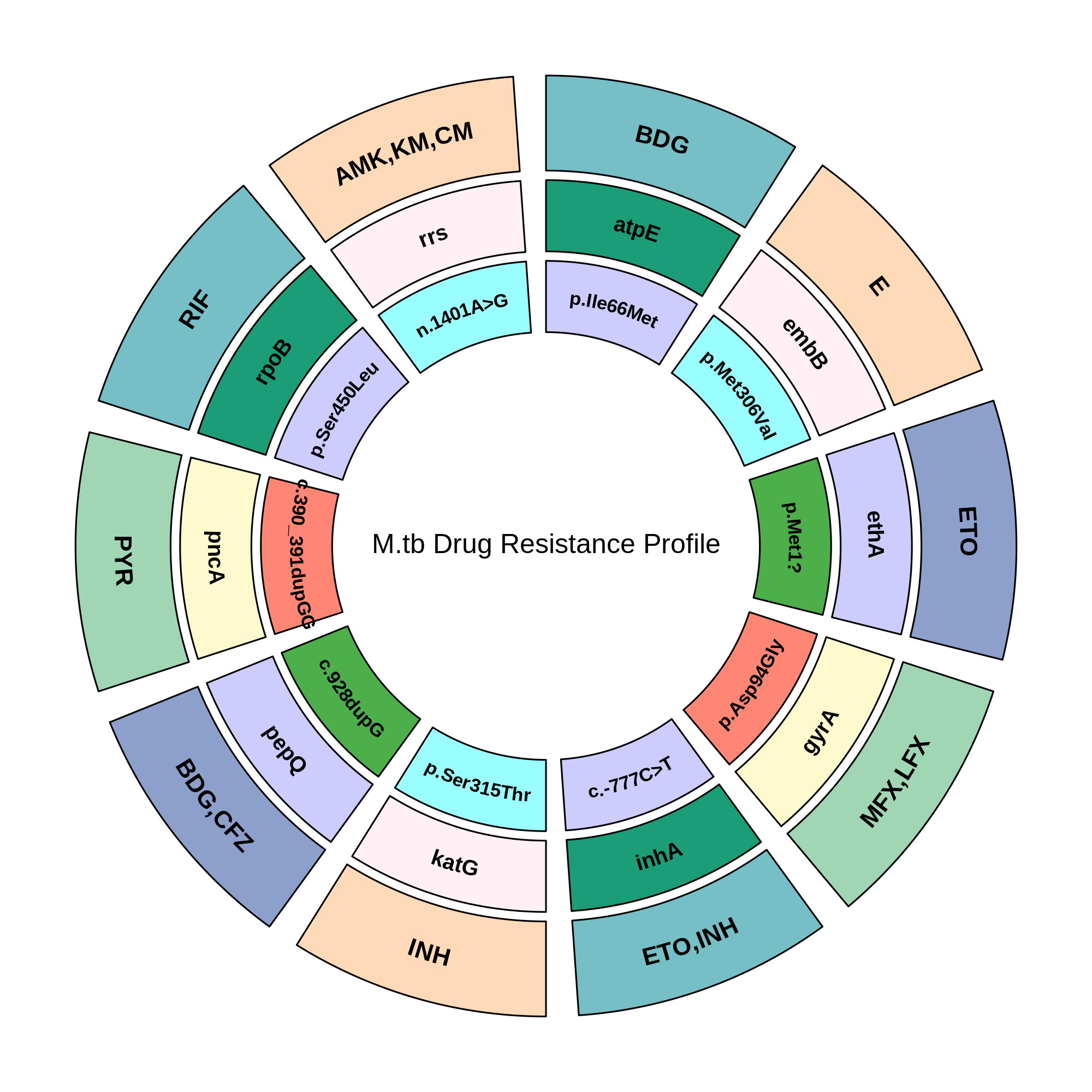
The diagram illustrates the association between Mycobacterium tuberculosis resistance genes and corresponding anti-TB drugs. Each segment represents a drug class linked to specific mutations. This integrated view highlights the genetic basis of resistance across first-line, second-line, and newer WHO recommended drugs, supporting identification to guide targeted TB therapy.
| Commercial Name | Cat No. | Pack Size | Platform |
|---|---|---|---|
| TB NGS test Kit | G710010-1 | 24 T | Illumina |
| G710010-2 | 96 T | Illumina | |
| G710010-3 | 96 T - EZY | Illumina - EZY | |
| TB NGS test Kit | G710010-4 | 24 T | MGI |
| G710010-5 | 96 T | MGI | |
| G710010-6 | 96 T - EZY | MGI - EZY | |
| TB NGS test Kit | G710010-7 | 24 T | Aviti |
| G710010-8 | 96 T | Aviti | |
| G710010-9 | 96 T - EZY | Aviti - EZY | |
| TB NGS test Kit | G710010-10 | 24 T | Thermo |
| G710010-11 | 96 T | Thermo | |
| G710010-12 | 96 T - EZY | Thermo - EZY |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.