Rapi-Q
Rapid Point of Care Testing Device
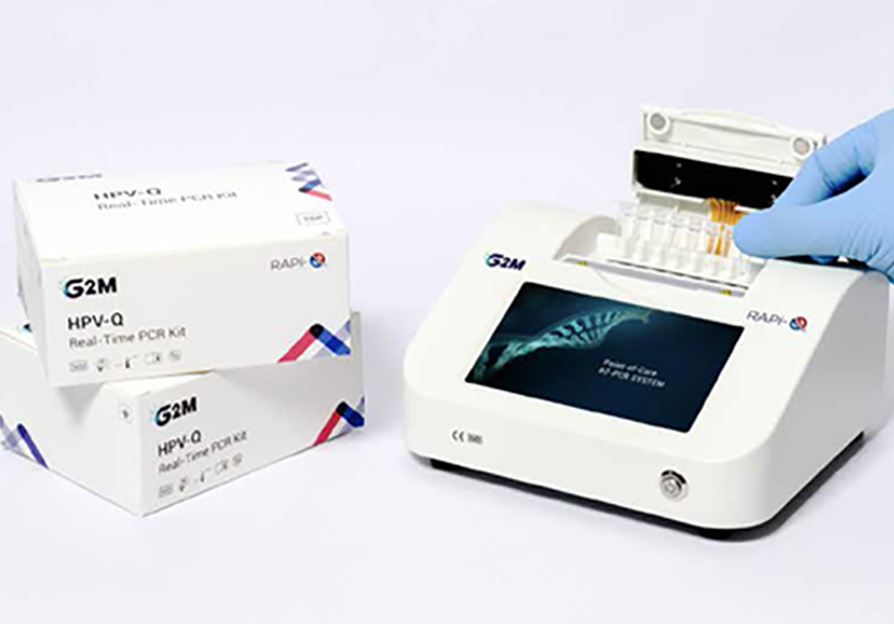
The Rapi-Q Rapid POC RT-PCR Testing Solution is a compact, lightweight, and user-friendly CE-IVD approved tool designed with an eight-well block and four fluorescent channels. It delivers results in approximately 30 minutes or more for up to 8 samples. This solution eliminates the need for technically skilled personnel by providing software-driven automated analysis, interpretation, and reporting. It supports qualitative and quantitative analysis, melting curve analysis, and genotyping.
| Model No. | Rapi-Q |
| Sample Capacity: | 8 rxn |
| Reaction Volume: | 10-50μl |
| MAX. Ramp Rate: | 6℃/s |
| Temperature Range: | 4-100℃ |
| Uniformity: | ±0.2℃ |
| Accuracy: | ±0.15℃ |
| Hot Lid Temperature: | 30 - 110℃ (Adjustable, default 105℃) |
| Excitation wavelength: | 455 - 650nm |
| Emission wavelength: | 510 - 750nm |
| Factory Calibrated Dyes: | F1: FAM/SYBR Green I, F2: HEX/VIC/JOE/TET, F3: ROX, F4: CY5/LIZ |
| Light Source: | 4 pcs highly efficient LED |
| Detection: | High sensitivity photoelectric detector |
| Dynamic Range: | 1-1010 Copies |
| Sensitivity: | 1 copy |
| Date Export Formats: | Report can be printed |
| Printing: | excel,cvs,txt |
| Dimension, Net Weight: | 180(L)×150(W)×90(H), 1.4 KG |
| Power: | 100 - 240 VAC, 50/60 Hz or compatible with battery backup |
|
Cat No. |
Commercial Name |
|
IG2M5001 |
Rapi-Q POC Rapid RT PCR System |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.