
The LeoNext cfDNA NIPT Solution offers a fully integrated, end-to-end workflow that supports laboratories across every stage of the testing process from cfDNA extraction and library preparation (with options for both automated and manual workflows) to data analysis and clinical reporting. By combining streamlined sample processing, robust sequencing library construction, and advanced bioinformatics, the system ensures reproducibility, scalability, and high-throughput capability, enabling laboratories to deliver consistent and reliable results with operational efficiency.
This platform provides a robust and sensitive approach for genome-wide fetal aneuploidy screening, supporting clinical laboratories in the delivery of high-quality prenatal genetic testing.
The LeoNext cfDNA Library preparation kit for NIPT is a CE-IVD certified in vitro diagnostic assay designed for comprehensive non-invasive prenatal testing (NIPT) utilizing next-generation sequencing (NGS). This assay screens for cell-free fetal DNA (cffDNA) extracted from maternal peripheral whole blood, enabling detection of genome-wide fetal chromosomal anomalies in pregnancies at ≥10 weeks of gestation.
Employing whole-genome sequencing, the assay is capable of identifying whole-chromosome aneuploidies as well as partial duplications and deletions across all autosomes, reporting sex chromosome aneuploidies (SCAs) as well as microdeletions.

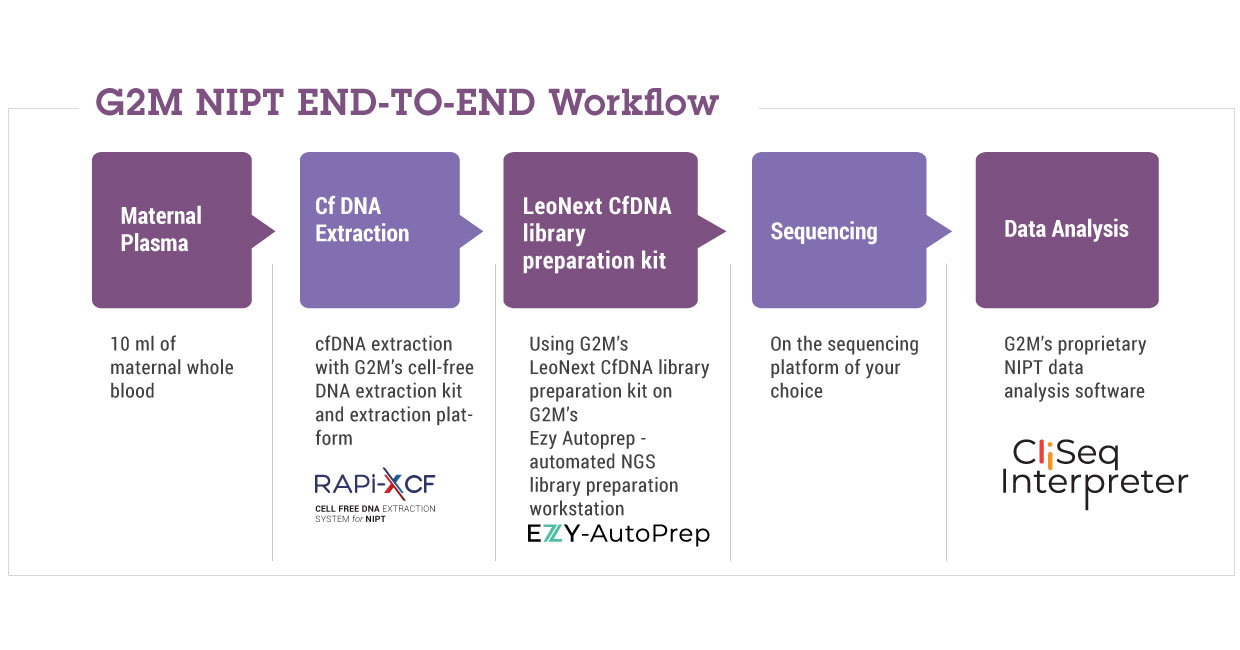
The LeoNext cfDNA NIPT solution delivers a complete NGS-based NIPT workflow, encompassing reagents and device for cfDNA extraction, kit and automation device for library preparation, instrumentation for automated library prep, a cloud-based software or an onsite server for secure data storage and analysis and generation of comprehensive qualitative result reports.
Designed for clinical laboratories, the Cliseq Interpreter software automates data processing, quality control, and statistical analysis to detect whole-chromosome aneuploidies, microdeletions and sex chromosome abnormalities. Results are presented through an intuitive PDF report that generates clear, comprehensive qualitative reports suitable for clinical decision-making.
| Commercial Name | Cat No. | Pack Size | Platform |
|---|---|---|---|
| LeoNext NIPT NGS Test Kit | G710022-1 | 48 T | Illumina |
| G710022-2 | 96 T | Illumina | |
| G710022-3 | 96 T - EZY | Illumina - EZY | |
| LeoNext NIPT NGS Test Kit | G710022-4 | 48 T | MGI |
| G710022-5 | 96 T | MGI | |
| G710022-6 | 96 T - EZY | MGI - EZY | |
| LeoNext NIPT NGS Test Kit | G710022-7 | 48 T | Aviti |
| G710022-8 | 96 T | Aviti | |
| G710022-9 | 96 T - EZY | Aviti - EZY | |
| LeoNext NIPT NGS Test Kit | G710022-10 | 48 T | Thermo |
| G710022-11 | 96 T | Thermo | |
| G710022-12 | 96 T - EZY | Thermo - EZY |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.