
Munich, Germany
17–21 April, 2026

Unlock deeper genomic insights with precision-engineered sequencing panels that enable comprehensive genetic profiling. Designed to deliver accurate detection of clinically relevant variants, these solutions support advanced diagnostics, hereditary screening, oncology applications, and personalized medicine.

Rapid, portable, and easy-to-use molecular testing systems that deliver lab-grade accuracy anywhere. Ideal for decentralized testing environments, clinics, screening centers, and on-site diagnostics where speed, accessibility, and accuracy are critical.

Flexible and scalable RT-PCR platforms that work with a wide range of reagents, assays, and workflows. Designed for clinical laboratories and research settings, these systems enable high-throughput, cost-effective molecular testing with complete operational freedom.

High-efficiency extraction kits and automated systems that ensure pure, consistent, and reliable recovery of DNA and RNA. Built for seamless workflow integration, they provide the ideal foundation for downstream molecular applications including PCR, sequencing, and clinical testing.
Explore our innovative, cutting-edge solutions in molecular diagnostics.
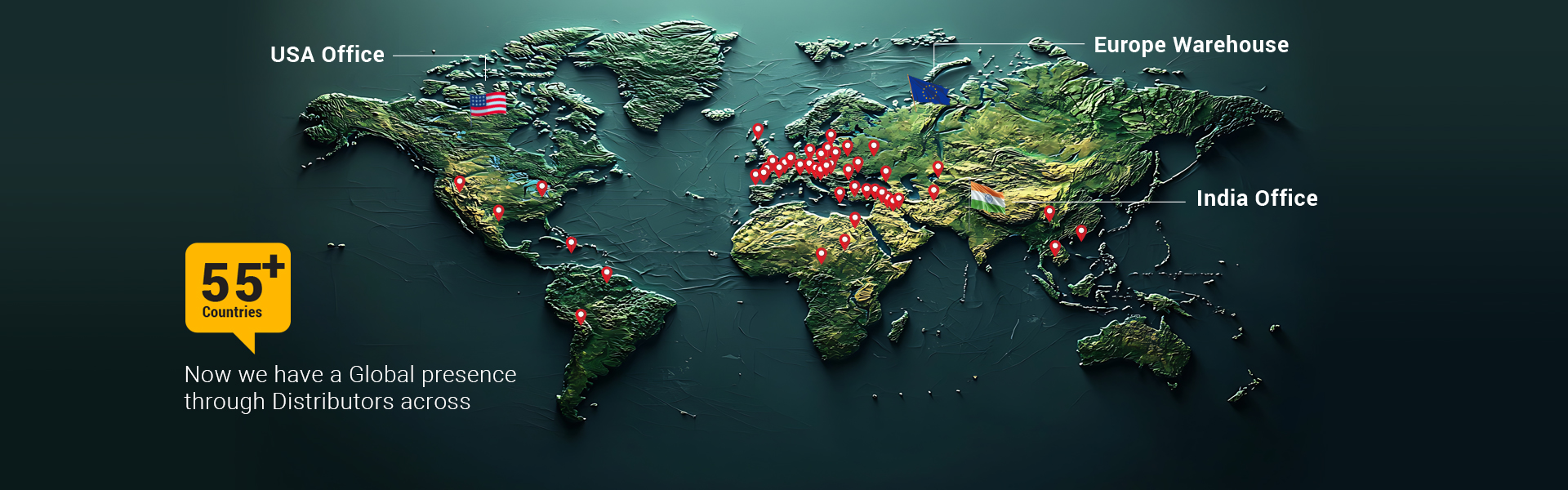
Global presence through Distributors across 55+ Countries
“37th European Congress of Pathology - Abstracts”

37th European Congress of Pathology - Abstracts
READ ARTICLE
Sexually transmitted infections (STIs) are a significant global health concern, affecting millions of people each year.
READ Blogs
Abstract 1903: Actionable mutational landscape in solid tumors: a case study of ovarian and colon cancer
READ ARTICLESince its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.