OncoCheck NGS assay is aimed to screen a range of disease causing genes to identify somatic mutations and germline mutations in DNA from FFPE, fresh tissue, and blood targeting 53 genes covering all the coding sequences enriched by hybridization capture-based target enrichment methodology. Genes are selected based on AMP/ASCO/CAP guidelines to uncover the coding region compiling to the size of 0.17 Mb.
Precision Enrichment Delivering Superior On-Target Performance
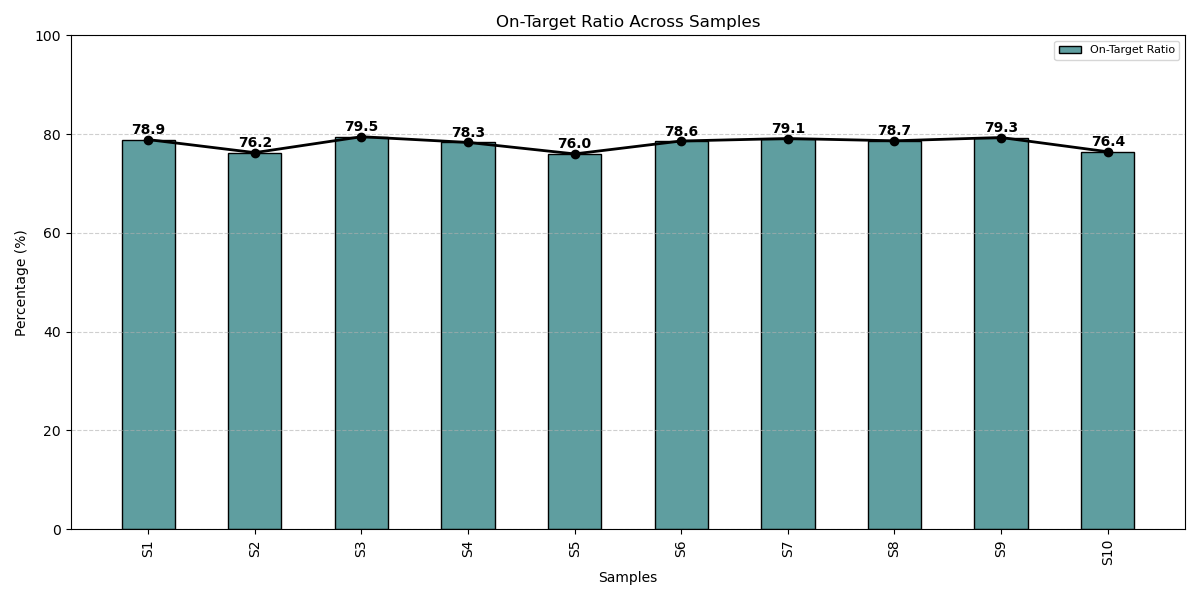
On-target ratio performance of the OncoCheck NGS Assay across samples maintains a high on-target alignment (ranging from 76.0% to 79.5%), reflecting exceptional assay design, optimized enrichment, and reliable sequencing accuracy for comprehensive genomic profiling.
Unwavering Uniformity in OncoCheck Assay Performance
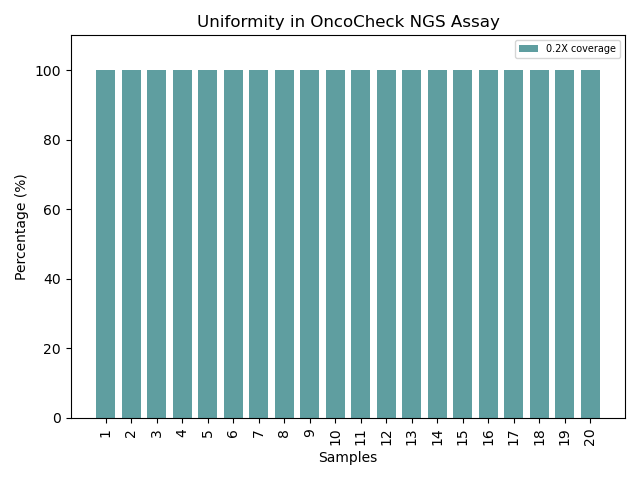
Uniformity of OncoCheck NGS Assay coverage across samples consistently achieves 100% coverage at 0.2X depth, demonstrating exceptional assay design and reproducibility for reliable genomic analysis.
| Commercial Name | Old Cat No. | New Cat No. | Pack Size | Platform |
|---|---|---|---|---|
| OncoCheck NGS Test Kit (HRR) | G2MOC01001 | G710002-1 | 24 T | Illumina |
| G2MOC01001 | G710002-2 | 96 T | Illumina | |
| G2MOC01001 | G710002-3 | 96 T – EZY | Illumina – EZY | |
| OncoCheck NGS Test Kit (HRR) | G2MOC01001 | G710002-4 | 24 T | MGI |
| G2MOC01001 | G710002-5 | 96 T | MGI | |
| G2MOC01001 | G710002-6 | 96 T – EZY | MGI – EZY | |
| OncoCheck NGS Test Kit (HRR) | G2MOC01001 | G710002-7 | 24 T | Aviti |
| G2MOC01001 | G710002-8 | 96 T | Aviti | |
| G2MOC01001 | G710002-9 | 96 T – EZY | Aviti – EZY | |
| OncoCheck NGS Test Kit (HRR) | G2MOC01001 | G710002-10 | 24 T | Thermo |
| G2MOC01001 | G710002-11 | 96 T | Thermo | |
| G2MOC01001 | G710002-12 | 96 T – EZY | Thermo – EZY |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.