Cancer is a life-threatening disease caused by the unchecked growth of abnormal cells, affecting nearly every part of the body but thanks to breakthroughs in genetic testing and medical technology, early detection is now more achievable than ever, offering new hope for timely and effective treatment. The Genes2Me CancerCheck 50 Assay offers a powerful 67 gene panel NGS test that utilizes advanced Next Generation Sequencing (NGS) technology to detect both germline and somatic mutations from blood or tumor tissue. By targeting genes most susceptible to cancerous mutations, this test enables early diagnosis and supports personalized treatment planning with high precision and reliability
Superior Consistency with Cancer Check 50 Assay
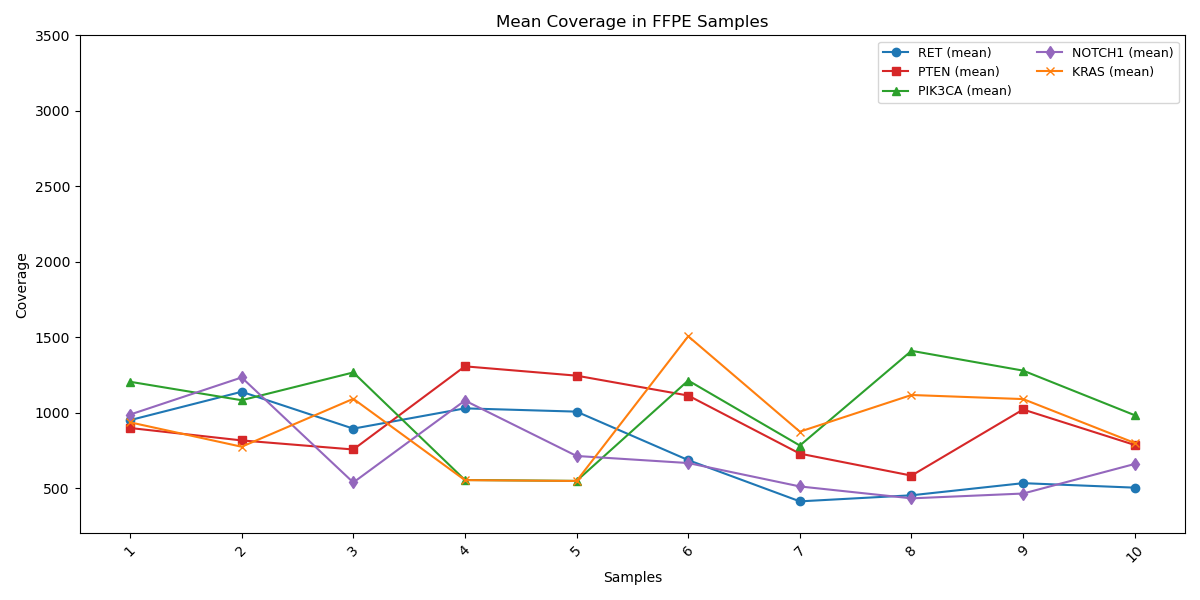
Mean coverage profiles of critical cancer-associated genes (RET, PTEN, PIK3CA, NOTCH1, and KRAS) reveal remarkable consistency and uniformity across all targets. This high level of coverage stability highlights the superior robustness and precision of the Genes2Me Cancer Check 50 Assay, ensuring reliable, high-confidence results for routine clinical testing.
Optimized Target Capture for Reliable Oncology Insights
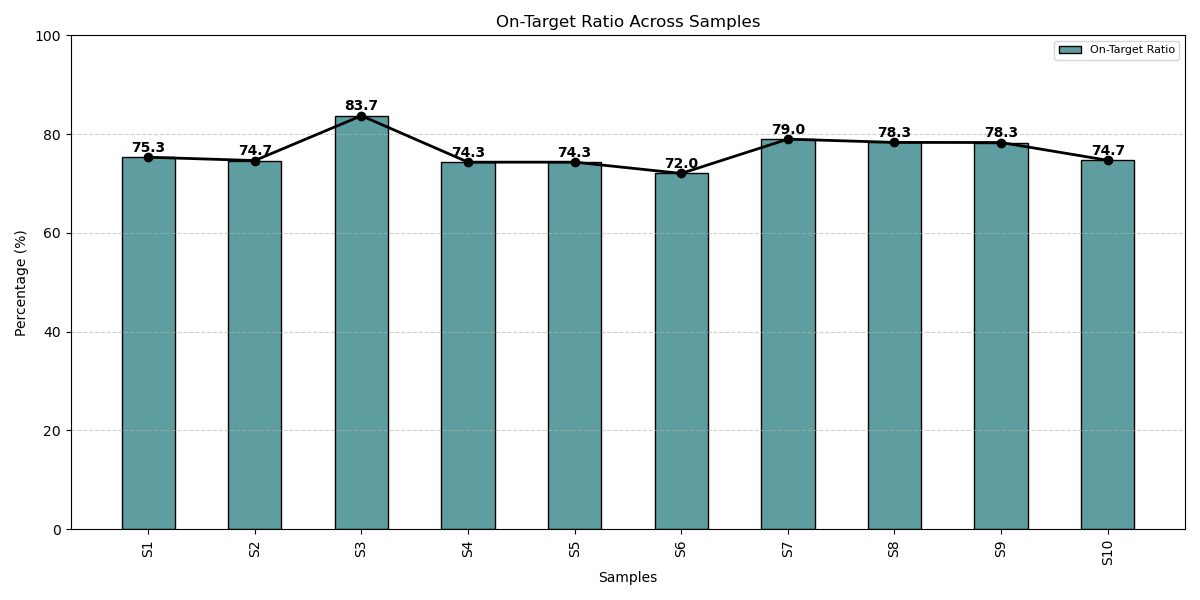
Consistent with over 75% on-target alignment across all cancer patient samples, our panel exemplifies exceptional design precision, optimized assay performance, and high-efficiency target capture delivering the clinically reliable data and scalable performance for impactful oncology outcomes.
| Commercial Name | Old Cat No. | New Cat No. | Pack Size | Platform |
|---|---|---|---|---|
| CancerCheck-50 NGS Test Kit | G2MCC03001 | G710004-1 | 24 T | Illumina |
| G2MCC03001 | G710004-2 | 96 T | Illumina | |
| G2MCC03001 | G710004-3 | 96 T – EZY | Illumina – EZY | |
| CancerCheck-50 NGS Test Kit | G2MCC03001 | G710004-4 | 24 T | MGI |
| G2MCC03001 | G710004-5 | 96 T | MGI | |
| G2MCC03001 | G710004-6 | 96 T – EZY | MGI – EZY | |
| CancerCheck-50 NGS Test Kit | G2MCC03001 | G710004-7 | 24 T | Aviti |
| G2MCC03001 | G710004-8 | 96 T | Aviti | |
| G2MCC03001 | G710004-9 | 96 T – EZY | Aviti – EZY | |
| CancerCheck-50 NGS Test Kit | G2MCC03001 | G710004-10 | 24 T | Thermo |
| G2MCC03001 | G710004-11 | 96 T | Thermo | |
| G2MCC03001 | G710004-12 | 96 T – EZY | Thermo – EZY |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.