Antimicrobial resistance is a global crisis and delays in detecting resistance patterns cost lives. The MDR-Q Real time PCR Kit enables real-time, point-of-care molecular screening of critical AMR genes directly from clinical samples. Designed for frontline use, it empowers hospitals, ICUs and surveillance teams to rapidly determine resistance signatures and guide targeted therapy even before culture results are available.
The AMR-Q POCT Kit is an advanced, lyophilized multiplex PCR-based diagnostic kit for rapid qualitative detection of 16 key antimicrobial resistance genes. The assay supports immediate clinical decision-making, helps antimicrobial stewardship programs, and enables healthcare systems to monitor and control drug-resistant infections in real time even in low-resource settings.
| Genes / Resistance Markers Detected | |
|---|---|
| Carbapenem resistance genes: blaNDM, blaKPC, blaOXA-48, others | |
| Vancomycin resistance: vanA, vanB | |
| Methicillin resistance: mecA, mecC | |
| Colistin resistance: mcr-1 | |
| Extended-spectrum β-lactamase (ESBL): blaCTX-M, blaTEM, blaSHV | |
| AmpC β-lactamase: ampC | |
| Sulfonamide resistance: sul1, sul2 | |
| Plasmid-mediated quinolone resistance (PMQR): qnr genes | |
| Internal Control (RNaseP): validates assay performance for each run | |
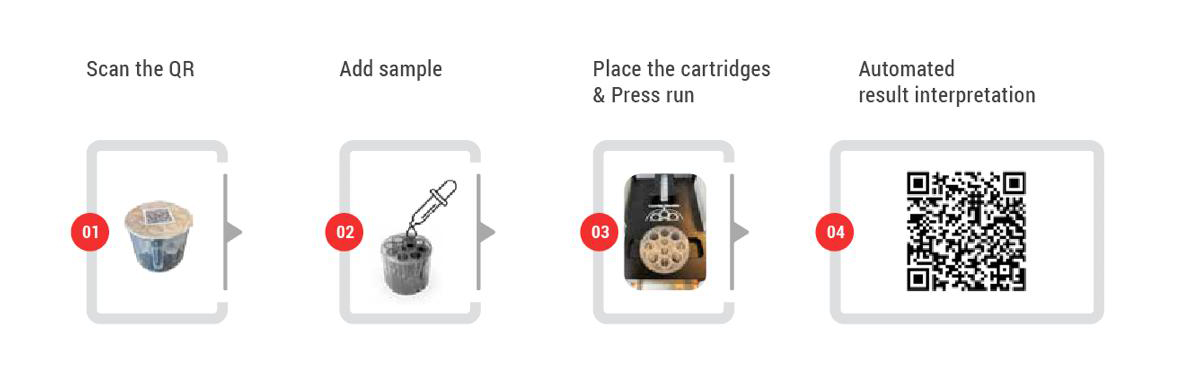
Collect & prepare clinical specime | Load into lyophilized PCR tube/cartridge | Insert cartridge into OnePCR device | Automated multiplex PCR amplification | Results displayed as Positive/Negative + Ct values
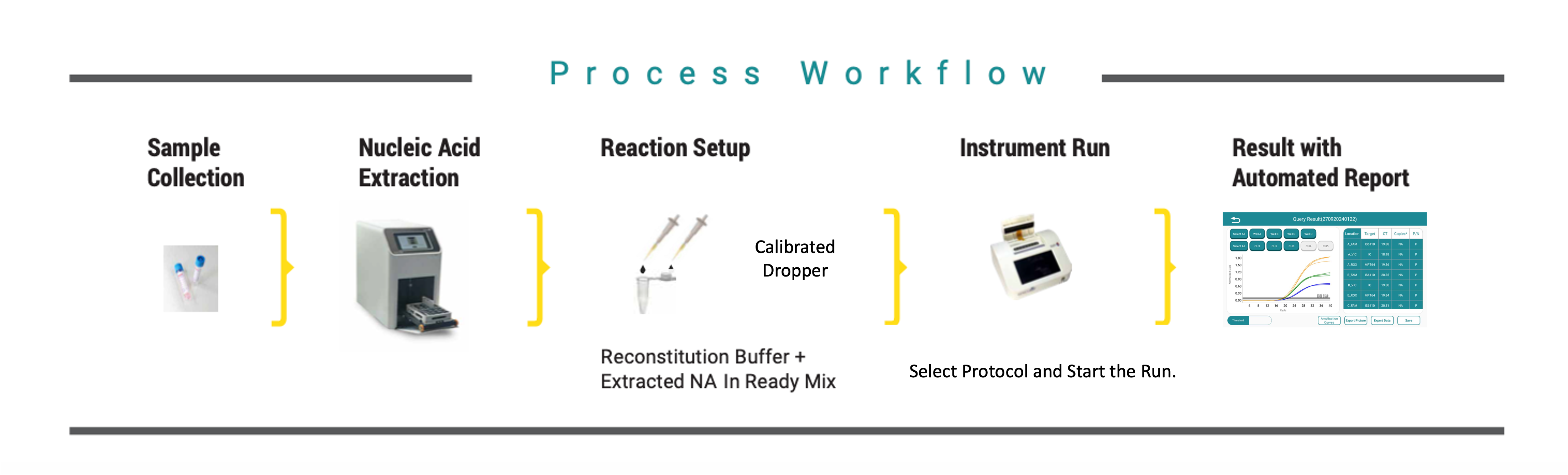
Perform DNA extraction using Rapi-X16 module | Reconstitute lyophilized reagents | Load into Rapi-Q system | Run AMR-Q program → view results in real-time
| Commercial Name | Cat No. | Pack Size | Compatible Instruments |
|---|---|---|---|
| AMR-Q Real time PCR Kit | G2M707521R - 24T | 24 Tests | OnePCR Ultra-Fast POC System |
| AMR-Q Real time PCR Kit | G2M707521R - 48T | 48T | Rapi-Q Portable PCR Device |
Download useful documents and technical information for the AMR-Q POCT Kit.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.