BRCA1 and BRCA2 are tumor suppressor genes, associated with inherited forms of breast and ovarian cancers. Mutations in both these genes are strongly implicated in hereditary breast, ovarian, and prostate cancers. Women carrying harmful BRCA1 variants face a 60 - 80% lifetime risk of breast cancer, while BRCA2 carriers face a 45–69% risk. Early and precise detection of these mutations is essential not only for confirming diagnosis but also for identifying at-risk individuals, guiding preventive strategies, family counseling, and targeted therapies.
The BRCA-Q Real-Time PCR Kit from Genes2Me is an advanced in vitro diagnostic solution for the qualitative detection of BRCA1 and BRCA2 mutations in clinical samples, including blood and FFPE tissues. With comprehensive mutation coverage, the BRCA-Q kit is designed to detect six high-risk mutations: five in the BRCA1 gene (185delAG, 300G [C61G], 2080delA, 4153delA, 5382insC) and one in the BRCA2 gene (6174delT). Independent primer-probe sets ensure mutation-specific amplification, while fluorescently labeled probes (FAM for BRCA targets, Cy5 for internal control) enable precise and parallel detection in a single workflow.
Built on highly sensitive real-time PCR technology, this kit empowers laboratories with accurate, fast, and reproducible results, enabling clinicians to make timely and informed decisions in cancer risk assessment, diagnosis, and treatment planning.

Built on sensitive real-time PCR technology to accurately detect low levels of mutated DNA with reliable reproducibility.
A Cy5-labeled internal control validates DNA quality, assay performance, and workflow integrity in every run, minimizing false-negative results.
Delivers accurate results within hours, supporting timely clinical decisions.
Ready-to-use reagents reduce hands-on time and minimize contamination risk, ensuring operational ease in routine testing environments.
Validated on leading real-time PCR systems including QuantStudio™ 5, Bio-Rad CFX96, Roche LightCycler® 480, and Genes2Me RapiCycler 96.
Reagents are stable for up to 12 months under recommended storage conditions, ensuring consistent and dependable performance.

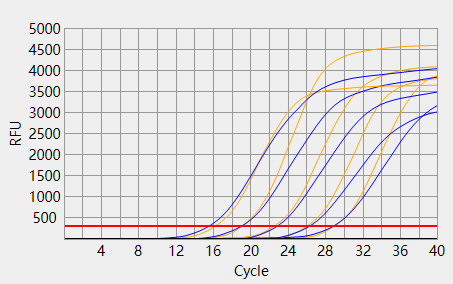
When the Ct value of the sample to be tested is outside the reference range and there is a typical S-shaped amplification curve, the test result is positive; When the Ct value of the sample to be tested is within the reference range, or the Ct value is outside the reference range but there is no typical S-shaped amplification curve, the test result is negative.
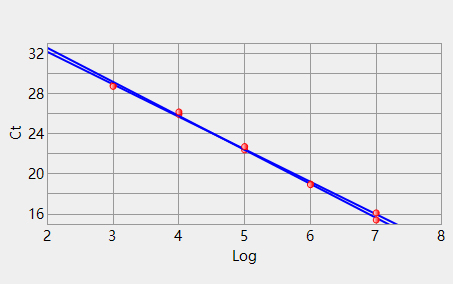
Channel 1 Slope: -3.392 Intercept: 39.372 Correlation: -0.998 Efficiency 3: 97.1430 Channel 3 Slope: -3.240 Intercept: 38.672 Correlation: -0.999 Efficiency 2: 103.52
| Commercial Name | Cat No. | Pack Size |
|---|---|---|
| BRCA-Q Real-Time PCR Test Kit | G2M802521 | 50 T |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.