The BRAF-Q Real-Time PCR Kit from Genes2Me is a next-generation molecular diagnostic solution designed for the qualitative detection of BRAF somatic mutations in DNA extracted from fresh, frozen, or FFPE (formalin-fixed paraffin-embedded) clinical samples. Mutations in the BRAF gene, particularly the V600E mutation, are among the most clinically relevant biomarkers in precision oncology. These mutations drive uncontrolled tumor growth and are strongly associated with multiple cancers, including melanoma, colorectal cancer, thyroid cancer, and lung cancer. Detecting BRAF mutations is therefore crucial not only for confirming diagnosis, but also for guiding targeted therapy decisions. By enabling oncologists to identify patients eligible for BRAF inhibitor therapies, the test directly contributes to better outcomes and improved survival rates.
Powered by advanced Real-Time PCR technology, this kit offers unmatched sensitivity, specificity, and reliability, giving clinicians and laboratories confidence in results that directly influence oncology diagnosis, prognosis, and treatment planning.
Detects low-level mutant DNA within a wild-type background using ARMS (Amplification Refractory Mutation System) and TaqMan probe chemistry. Demonstrates >99% sensitivity and specificity, ensuring accurate mutation detection.
Streamlined workflow delivers precise mutation status within hours, supporting rapid clinical decision-making.
Integrated BRAF Internal Control validates DNA quality, assay workflow, and reagent performance—minimizing false-negative results.
Optimized for fresh tissue, frozen samples, and FFPE specimens, enabling routine use across diverse clinical sample types.
Ready-to-use reagents reduce hands-on time and contamination risk. Compatible with major real-time PCR platforms including QuantStudio™ 5, Bio-Rad CFX96, Roche LightCycler® 480, and RapiCycler 96 (Genes2Me).
Manufactured under stringent quality standards for extended shelf stability and robust performance. Supported by Genes2Me’s expert technical team, ensuring reliable end-to-end assistance for laboratories.

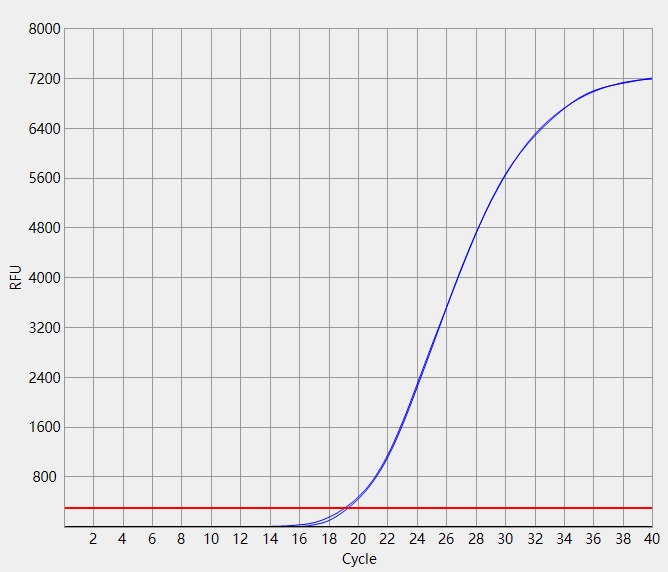
When the Ct value of the sample to be tested is outside the reference range and there is a typical S-shaped
amplification curve, the test result is positive;
When the Ct value of the sample to be tested is within the reference range, or the Ct value is outside the reference
range but there is no typical S-shaped amplification curve, the test result is negative.
| Commercial Name | Cat No. | Pack Size |
|---|---|---|
| BRAF-Q Real-Time PCR Kit | G2M708521 | 25 T |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.