Accurate differentiation between Mycobacterium tuberculosis (MTB) and Non-Tuberculous Mycobacteria (NTM) — enabling precise diagnosis and appropriate treatment decisions.
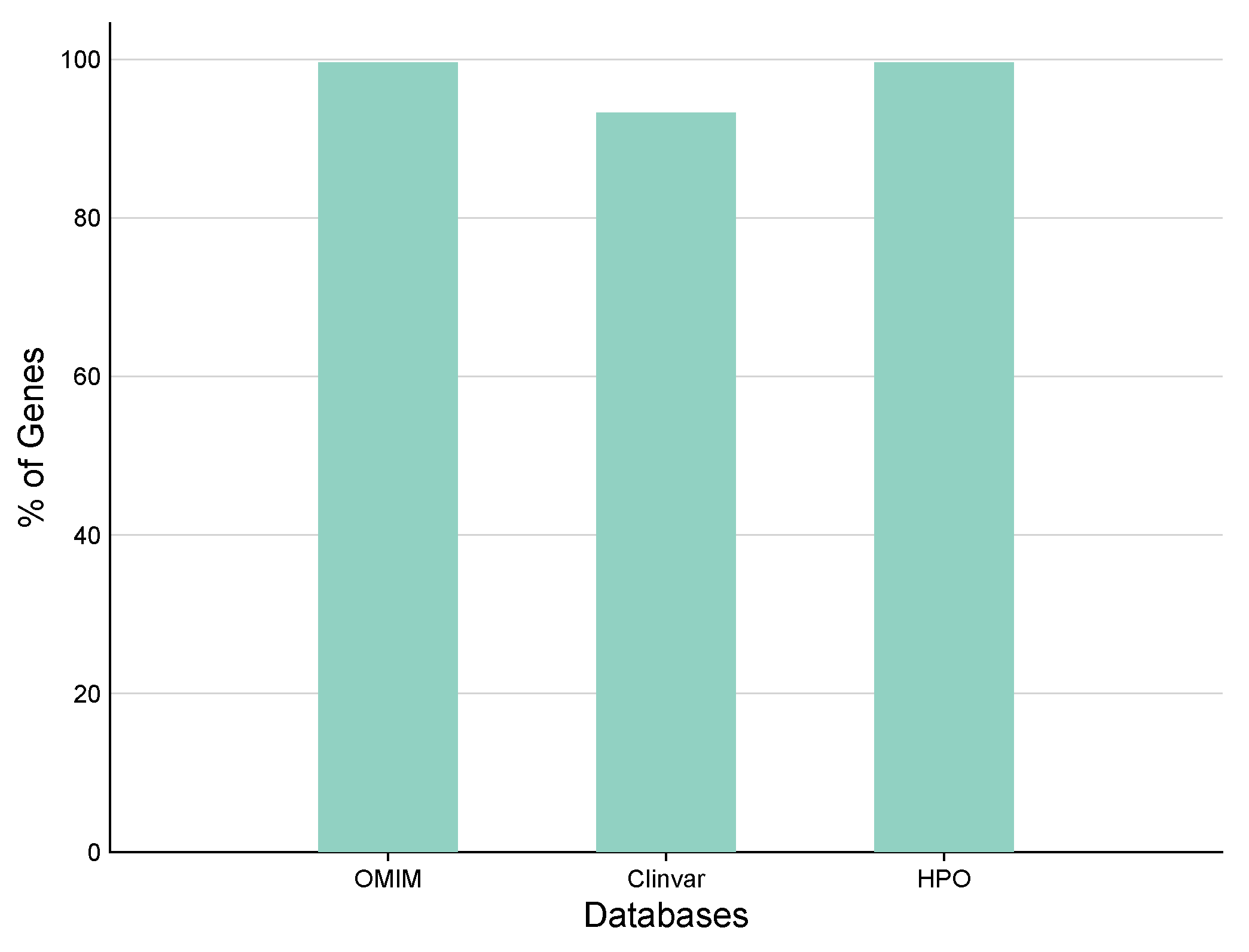
3H-Q Real Time PCR Kit Panel is designed to detect different germline mutations by screening nearly 21,500 clinically relevant genes (coding regions) and mitochondrial genome. G2M WES panel’s automation-friendly workflow enables identification of significant SNV, InDels and CNV spanning a target region of 38.2 Mb. The panel covers mutations from all the widely used major databases (ClinVar, OMIM, 1000 Genomes, gnomAD, dbSNP). Genes 2Me Whole Exome Sequencing (WES) Panel is designed to detect different germline mutations by screening nearly 21,500 clinically relevant genes (coding regions) and mitochondrial genome.

M WES panel’s automation-friendly workflow enables identification of significant SNV, InDels and CNV spanning a target region of 38.2 Mb. The panel covers mutations from all the widely used major databases (ClinVar, OMIM, 1000 Genomes, gnomAD, dbSNP). Genes 2Me Whole Exome Sequencing (WES) Panel is designed to detect different germline mutations by screening nearly 21,500 clinically relevant genes (coding regions) and mitochondrial genome. G2M WES panel’s automation-friendly workflow enables identification of significant SNV, InDels and CNV spanning a target region of 38.2 Mb. The panel covers mutations from all the widely used major databases (ClinVar, OMIM, 1000 Genomes, gnomAD, dbSNP).
| List of Diseases category assessed by PML-RARA Real Time PCR Kit* | |
|---|---|
| Disease Class | List Of Diseases |
| Cardiac disorders | Dyslipidemia, Aortopathy, Congenital heart defect, cardiovascular diseases |
| Dermatological disorders | Ectodermal dysplasia, Albinism, Xeroderma pigmentosum, Ichthyosis |
| Endocrinological disorders | Pancreatitis, Premature ovarian failure, Adrenal hyperplasia, Hyperparathyroidism |
| Bone disorders | Arthrogryposis, Osteopetrosis, Cleft lip palate, Amelogenesis imperfecta |
| Immunological disorders | Immune dysregulation, Defects in intrinsic and innate immunity |
| Hepatological disorders | Polycystic liver disease, Cholestasis, Congenital hepatic fibrosis |
| Hematological disorders | Bleeding & Thrombotic disorder, Bone marrow failure, Anemia |
| Metabolic disorders | Aminoacidopathies, Purine/Pyrimidine disorders, Creatine biosynthesis disorders |
| Eye disorders | Ectopia lentis, Retinoblastoma, Corneal dystrophy, Optic atrophy |
| Pulmonological disorders | Bronchiectasis, Cystic fibrosis, Primary ciliary dyskinesia |
| Neurological disorders | Neuromuscular disorders, Autism, Seizures & Brain abnormalities, Neurodegenerative disorders |
| Oncological disorders | Hematological malignancy, Brain cancer, Colorectal cancer, Breast cancer, Ovarian cancer |
| PML-RARA Real Time PCR Kit? | |
|---|---|
| Unclear Diagnosis: When a patient’s symptoms do not lead to a definitive diagnosis or phenotype of a suspected genetic disorder. | |
| Delayed Diagnosis Impacting Quality of Life: If a delayed or uncertain diagnosis could significantly affect the patient’s quality of life, WES may help expedite the process.. | |
| Ineffective Stepwise Diagnostics: In cases where a step-by-step diagnostic approach would be time-consuming and costly, WES can be a more efficient alternative. | |
| Lack of Plausible Diagnosis: When a physician is unable to propose a clear diagnosis based on the symptoms, WES can help uncover underlying genetic causes. | |
| No Other Diagnostic Options: When no other available diagnostic techniques can confirm the condition or end the diagnostic odyssey, WES might provide the answers needed | |
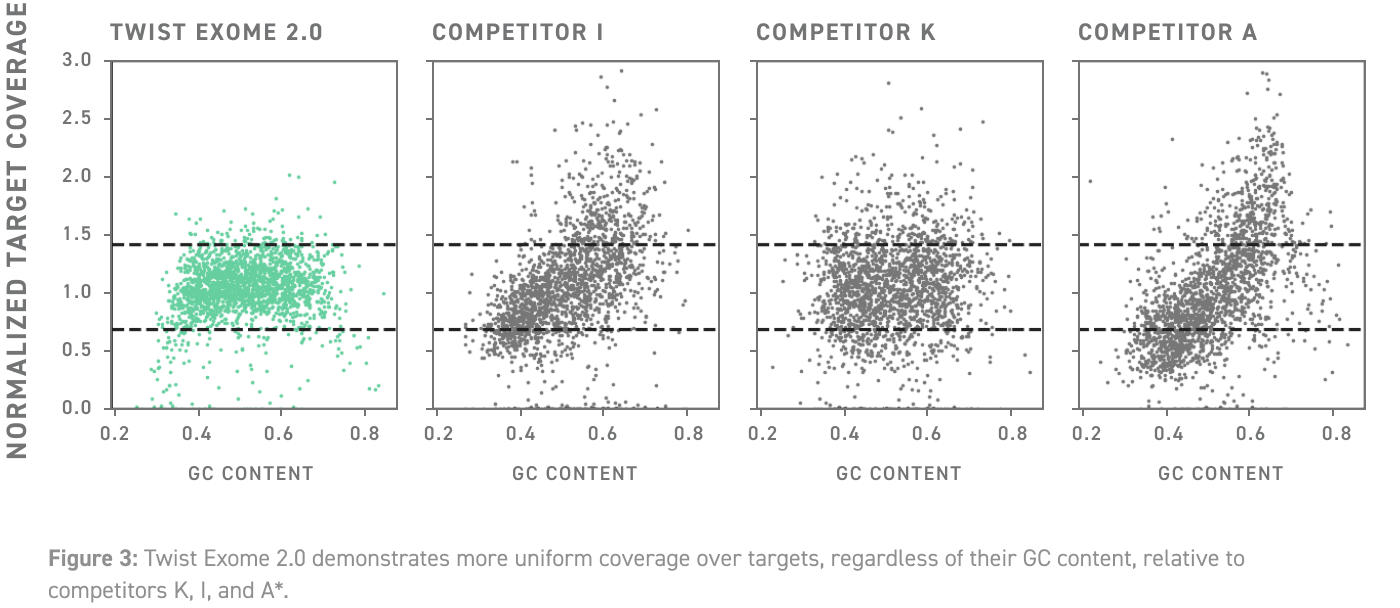
| Features | Illumina | MGI | Thermofisher |
|---|---|---|---|
| Coverage uniformity | 96% | 96% | 87% |
| Precision | 94% | 94% | 87% |
| Reproducibility | 97% | 97% | 93% |
| Sensitivity | 94% | 94% | 87% |
| On Target Ratio | 85-95% | 85-95% | 80-85% |
.jpg)
.jpg)
Manage runs, analyze, store and share data in a centralized environment with no command line interface (CLI) or specialized coding skills required.
Reduce manual touchpoints; instrument integration automatically streams sequencing data to the cloud and kicks off analysis.
Access run data anywhere, anytime. Cloud-based application improves access and simplifies operations with real-time monitoring.
Collaborate easily with secure, audit-controlled data management and sharing without requiring file downloads.
Accelerate analysis with a curated and intuitive app menu, including award-winning DRAGEN secondary analysis applications.
Scale storage up or down as needed—no IT support required. Supports long-term data archiving and optimized savings.
| Ordering Information | |
|---|---|
| Commercial Name | Cat No. |
| Clinical Exome Sequencing Expanded Panel (Whole Exome Sequencing) | G2MCES07001(WES)-ill |
| Clinical Exome Sequencing Expanded Panel (Whole Exome Sequencing) | GMCES07001(WES)-MG |
| Clinical Exome Sequencing Expanded Panel (Whole Exome Sequencing) | G2MCES07001(WES)-TF |
Download useful documents and technical information for the HCV-Q RT-PCR Kit.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.