MagNXT Blood DNA Extraction Kit is designed for the isolation and purification of genomic DNA from clinical blood samples including whole blood, plasma, serum, and buffy coat/lymphocytes. The kit uses magnetic bead-based purification technology combined with enzymatic lysis to ensure efficient recovery of high-quality DNA suitable for downstream molecular applications.
Kit components: MagPure Particles | Buffer LB | Wash BW1 | Wash BW2 | Proteinase K | Proteinase Dissolving Buffer | Binding Buffer | Buffer AE
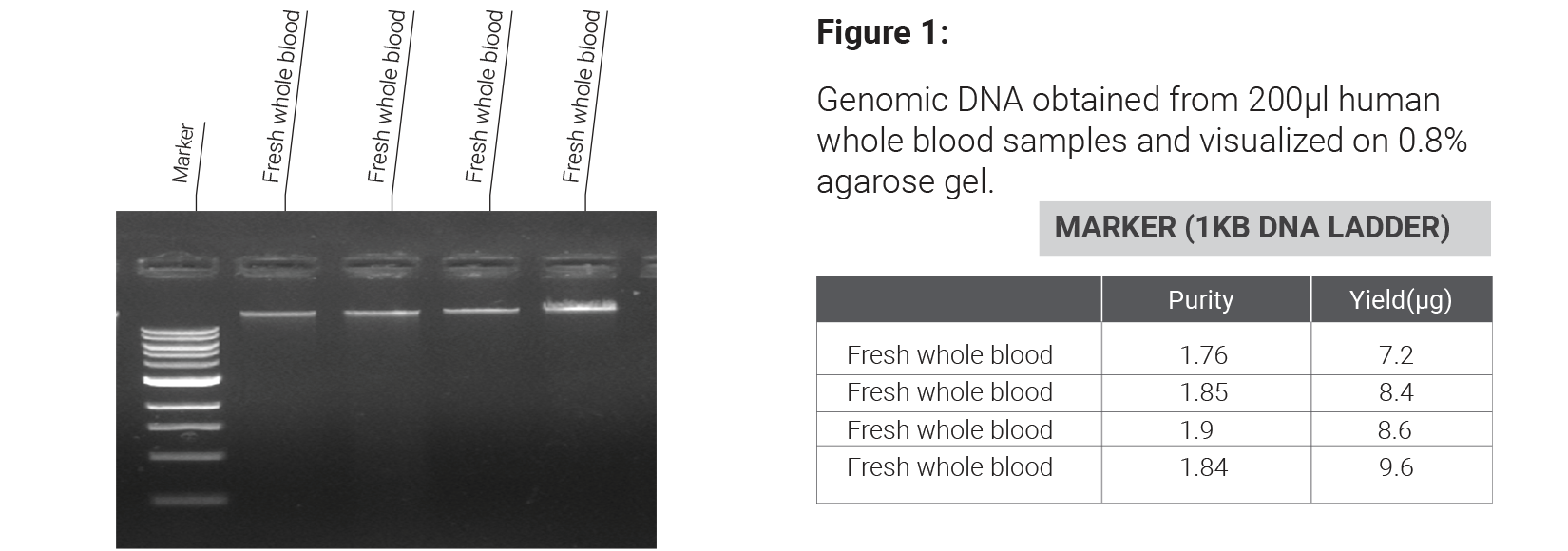
MagNXT Blood DNA Extraction Kit is based on superparamagnetic particle purification technology, enabling rapid, reliable, and reproducible DNA extraction from blood-derived samples. The method involves cell lysis, protein digestion using Proteinase K, selective binding of DNA to magnetic beads, washing to remove contaminants, and elution of purified DNA using a low-salt buffer. The kit can be processed Manually or on open-ended automated liquid handling platforms enabling reliable DNA extraction without the use of phenol/chloroform extraction or alcohol precipitation steps, and making it suitable for routine clinical laboratory workflows as well as research laboratories.

| Format: | Paramagnetic Beads |
| Technology: | Magnetic Bead Base |
| Sample Type: | Whole Blood (200µl), Serum, Plasma, Lymphocytes/Buffy coat |
| Yield: | ≥7–12 µg |
| Target: | Genomic DNA |
| A260/280: | 1.6–1.9 |
| Available Pack Sizes: | 50 T, 250 T, 96 T, 192 T, 480 T |
| Throughput Compatibility: | Manual and automation both |

| Commercial Name | Cat No. | Pack Size |
|---|---|---|
| MagNXT Blood DNA Extraction Kit | G2M182121-50T | 50 T |
| MagNXT Blood DNA Extraction Kit | G2M182121-250T | 250 T |
| MagNXT Blood DNA Extraction Kit | G2M182121-96T | 96 T |
| MagNXT Blood DNA Extraction Kit | G2M182121-192T | 192 T |
| MagNXT Blood DNA Extraction Kit (RX) | G2M182121RX-480T | 480 T |
| MagNXT Blood DNA Extraction Kit (TK) | G2M182121TK-480T | 480 T |
| MagNXT Blood DNA Extraction Kit (MG) | G2M182121MG-480T | 480 T |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.