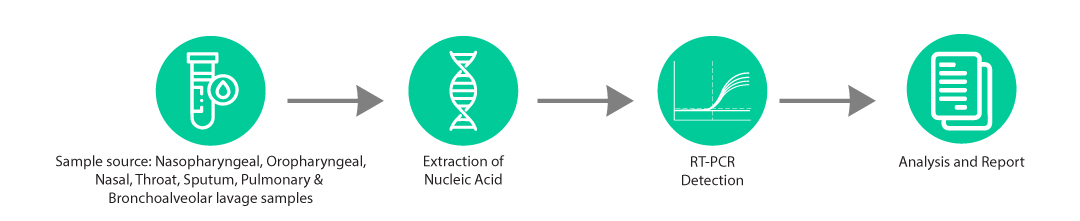
The B. Pertussis-Q kit is an in-vitro molecular diagnostic test, based on real-time PCR technology, for qualitative detection of Bordetella pertussis bacteria specific DNA in clinical samples which is also commonly known as whooping cough, an upper respiratory infection.
B. Pertussis Specific Primer and Probe Mix | Positive Control Template | Positive Control-2 (H3N2) | RNaseP Primer & Probe | q-PCR Master Mix | Reconstitution Buffer | RNase/DNase free water

Compatibility with all Common Platforms

Reliable Result with Endogenous Internal Control
Extensively Validated on wide variety of Clinical Specimens

Quick
Turnaround Time

High Sensitivity & Specificity

CE-IVD Certified Diagnostic Test
● Compatible with a wide range of 4 or 6 Fluorescence Channel Real-Time PCR devices and Genes2Me RapiCycler 96 Real Time PCR System
● Compatible with the Genes2Me’s RAPi-Q and RAPi-Q HT Point-of-Care (POC) RT-PCR Systems
CAT # |
DESCRIPTION |
UNITS |
G2M706821 |
B. Pertussis-Q Real Time PCR Kit |
100 Rxn |
G2M706821 |
B. Pertussis-Q Real Time PCR Kit |
50 Rxn |