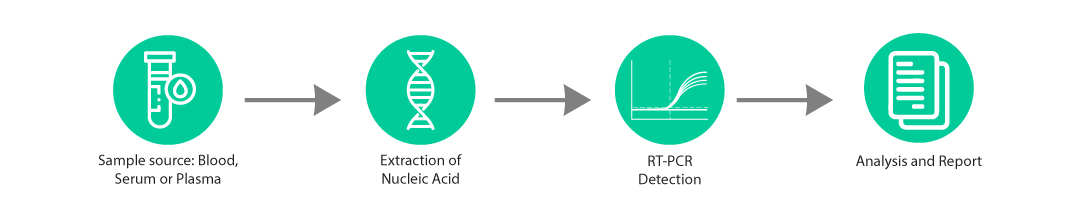
The Zika-Q kit is an in-vitro molecular diagnostic test, based on real-time PCR technology, for qualitative detection of zika virus specific RNA in clinical samples.
Zika Specific Primer and Probe Mix | Positive Control Template | RNaseP Specific Primer and Probe | q-PCR Master Mix | Reconstitution Buffer | RNase/DNase free water

Compatibility with all Common Platforms

Reiable Result with Endogenous Internal Control
Extensively Validated on wide variety of Clinical Specimens

Quick
Turnaround Time

High Sensitivity & Specificity

CE-IVD Certified Diagnostic Test
● Compatible with a wide range of 4 or 6 Fluorescence Channel Real-Time PCR devices and Genes2Me RapiCycler 96 Real Time PCR System
● Compatible with the Genes2Me’s RAPi-Q and RAPi-Q HT Point-of-Care (POC) RT-PCR Systems
CAT # |
DESCRIPTION |
UNITS |
G2M262621 |
Zika-Q Real Time PCR Kit |
100 Rxn |
G2M262621 |
Zika-Q Real Time PCR Kit |
50 Rxn |