The CoVFlu-II kit is an in-vitro molecular diagnostic test, based on real-time PCR technology, for qualitative detection and differentiation of SARS-CoV-2, Influenza A and Influenza B virus specific RNAs in clinical samples.
Influenza A&B Specific Primer and Probe | SARS-CoV-2 (RdRp and N gene) Specific Primer and Probe | RSV Specific Primer and Probe | RNaseP Control Specific Primer and Probe | Positive Control Template | OneStep qRT-PCR Master Mix | Reconstitution Buffer | Negative Control | RNase/DNase free Water

Compatibility with all Common Platforms

Reiable Result with Endogenous Internal Control
Extensively Validated on wide variety of Clinical Specimens

Quick
Turnaround Time

High Sensitivity & Specificity

CE-IVD Certified Diagnostic Test
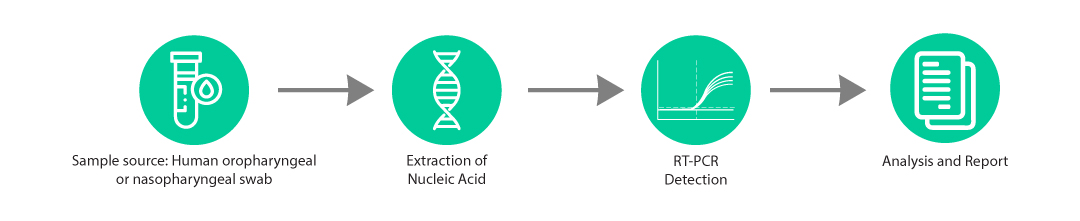
● Compatible with a wide range of 4 or 6 Fluorescence Channel Real-Time PCR devices and Genes2Me RapiCycler 96 Real Time PCR System
● Compatible with the Genes2Me’s RAPi-Q and RAPi-Q HT Point-of-Care (POC) RT-PCR Systems
CAT # |
DESCRIPTION |
UNITS |
G2M709821 |
CoVFlu-II One Step RT-PCR Kit |
100 Rxn |
G2M709821 |
CoVFlu-II One Step RT-PCR Kit |
50 Rxn |